In Vitro Cell Viability Service
Summary
Toxicity is a crucial factor to test when evaluating test compounds as potential drug candidates. In most cases, test compounds should not be detrimental to the health of the cells they are treating. In order to evaluate the toxicity of test compounds, BioAssay Systems offers three different methods of measuring in vitro cell viability.
After treatment with test compounds, cell viability can be evaluated by the following methods:
After treatment with test compounds, cell viability can be evaluated by the following methods:
- The levels of intracellular ATP as an indicator of healthy cells;
- Levels of Lactate Dehydrogenase (LDH) excreted into the cell media from damaged cells as an indicator of toxicity;
- Cellular reduction capacity using either a fluorescent or colorimetric dye as a measure of viability.
Method
The cell line of interest is plated at the specified cell density and the respective positive controls, negative controls, and treatments are added to the media. After incubation with the treatments for the specified time, the respective reagent for the desired test method is added to the wells. The wells are then read via luminescence, fluorescence, or absorbance either immediately or after further incubation depending on the test method.
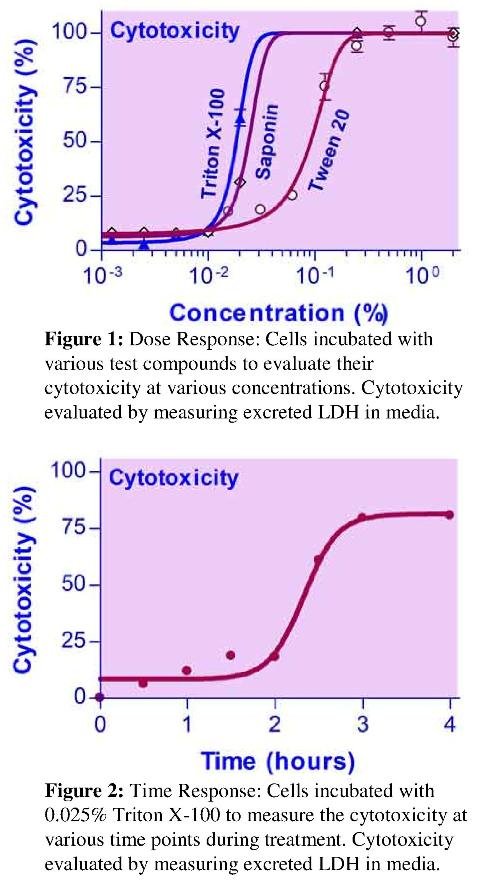
Compounds can be evaluated for dose response, where the cytotoxicity is evaluated at various concentrations for a set treatment duration, or for time response, where cytotoxicity of a specific concentration is measured at time points throughout the course of treatment.
Our Services
- Depending on the project, 1-10 mg compound is needed.
- No structure information is required.
- If compound is in DMSO, 10mM or higher concentration is preferred.
- Report will be available within 2 to 15 days.
Please email or call us at 1-510-782-9988 x 2 to discuss your service needs.